
Building a scalable ePRO platform
Patients struggle to share their experience with HCPs
During medical appointments, patients often struggle to precisely describe their experience and outcomes between appointments to their healthcare professionals (HCPs). This leads to patients feeling less empowerment and engagement in their treatment, and HCPs being faced with limited available data for their decision making. In this context, the Global Digital Health team of a leading pharmaceutical company reached out to us to discuss how to build a scalable ePRO
platform offering a premium patient experience. The goal: enabling patients’ use of ePROs in an easy and accessible way to enable them to track their symptoms and outcomes longitudinally for significantly improved patient-HCP interactions.
A user-centric approach
Our approach was focused on leveraging existing ePROs that are already scientifically validated and available in many countries and languages. The focus was to transform these ePROs into an engaging tool to create longitudinal records for patients, focusing on a few key areas: a very smooth flow of the questionnaires, accessibility from any device, easy onboarding into the account, and structured and impactful reports which can easily be shared
with HCPs. The reports shall then become a core part of patients’ preparation of their discussion with their HCP about the progression of their condition.
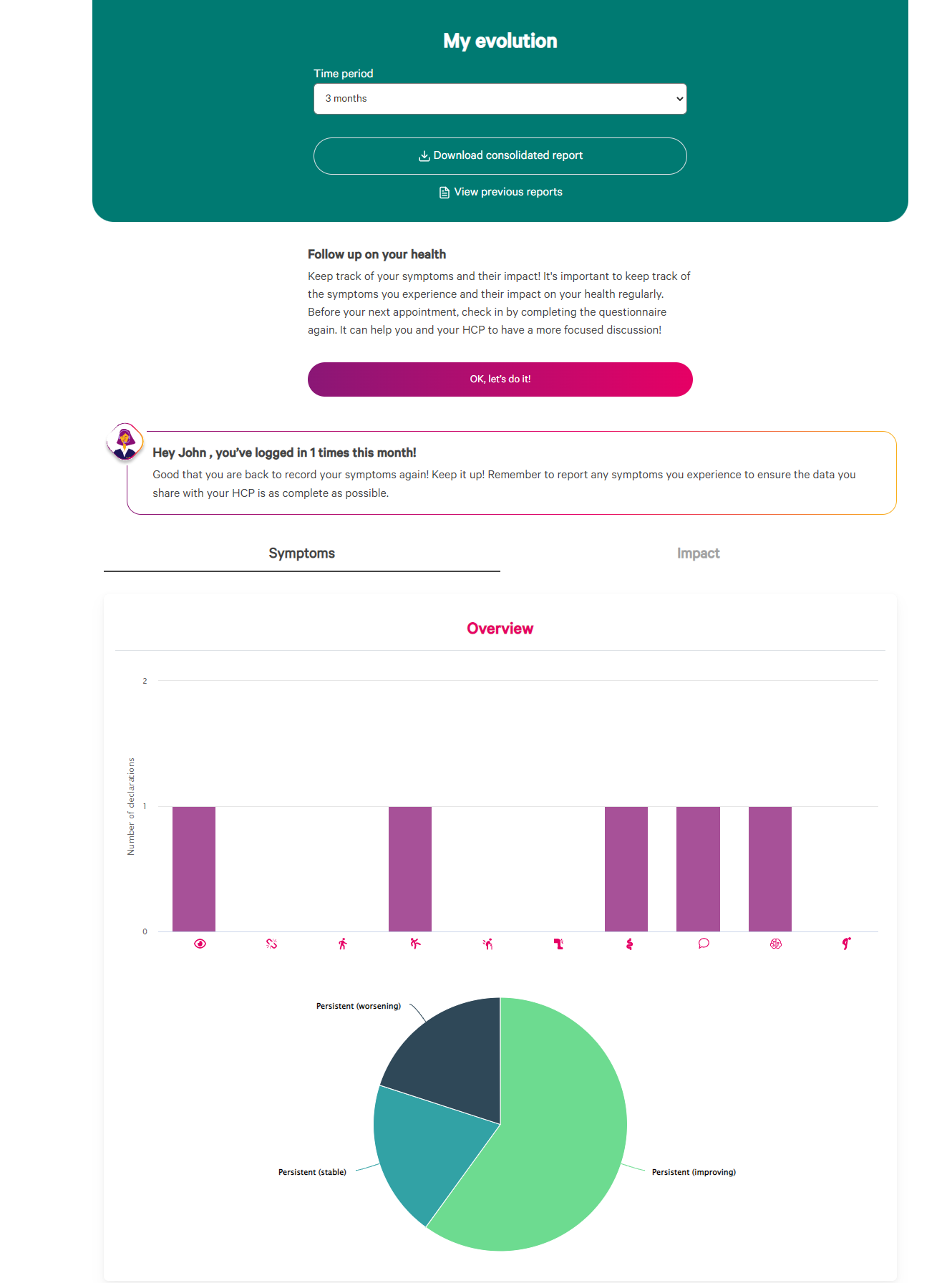
Once an account is generated, patients are encouraged to complete the questionnaire regularly through personalized reminders. They have access to a personalized dashboard to see their data, and consume personalized content to help them manage their disease.
Testimony
Figures
Results
Conclusion
Finally, new features are being developed to enhance patient engagement, such as the integration of our SPUR™ behavioral diagnostic tool and smart reminders.
See also
A multiple-cohort analysis of the SPUR 6/24 patient-reported adherence tool
Clinical Ink and Observia: Pioneering Personalized Patient Engagement in Clinical Trials
By your side in 2024: Happy Holidays from Observia!
Need more information?
A burning question, a specific request, a great project to share with us? Get in touch, our teams are here to help!










