
Building a holistic blueprint for a hybrid and scalable PSP
Designing a patient support program adaptable to multiple markets
Our client is a global research-driven life science company, entering the area of dermatology with a new treatment. To support their strategy, they needed to reinforce their position as a patient-centric and innovative leader in this therapeutic area; they chose to do so by developing a Patient Support Program (PSP) available to all patients suffering from the skin condition they are addressing
Our mission was to build the blueprint for this PSP in a collaborative way by understanding the patients’ needs, defining the functionalities, technical necessities, and deployment plans, while ensuring that the value proposition was aligned with the needs of every stakeholder involved.
This blueprint also needed to support a multi-year product rollout strategy, and cover holistically the whole patient journey, from pre-diagnostic to post-treatment. Finally, the challenge was to build a global blueprint that could be easily deployed across affiliates.
A structured guide to building a PSP
Based on the client’s market research, Observia has developed a strategic and operational blueprint to guide their local affiliates into launching a modular PSP capable of answering market specificities. This has been done in several phases:
1) review and consolidation of in-house knowledge,
2) development of an efficient mechanism to engage local teams,
3) gathering of patients' needs and expectations,
4) generation of the PSP’s objectives, scope, strategy, IT specifications, functionalities and KPIs
5) final validation.
The strategic blueprint contains key elements such as personalisation and customisation strategy, enrolment and engagement strategy, channel and content strategy, functionality requirements etc.
The operational blueprint contains practical guidance such as solution description, value proposition, baseline offering, content, data collection, user flow, PSP architecture and regulatory aspects.
- High level of operational detail with real mockups and user flows
- Hybrid deliverable: half strategic, half operational
- Simultaneous, co-creation between global, 4 key markets and patients
Testimony
Methods
Results
This method has resulted in a well-defined value proposition for the client to become a patient-centric player.
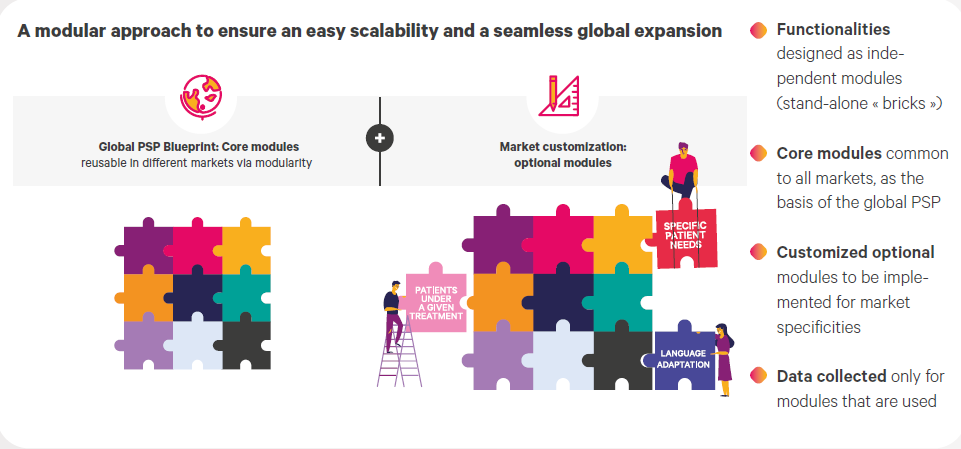
The modular design of PSP will ensure an easy scalability and a smooth implementation in any local market.
Conclusion
The use of Observia’s 4-pillar in-house methodology allowed for a smooth collaboration with the client’s teams, at global and affiliate levels. Observia’s extensive insights have contributed to building a ready-to-use blueprint which will be at the basis of a holistic patient support program aiming at deeply engaging patients and answering their needs. Our modular conception of this blueprint will help deploying the PSP at scale and make it possible for the solution to evolve in line with future understanding of this skin pathology, as well as stakeholders’ needs.
See also
A multiple-cohort analysis of the SPUR 6/24 patient-reported adherence tool
Clinical Ink and Observia: Pioneering Personalized Patient Engagement in Clinical Trials
By your side in 2024: Happy Holidays from Observia!
Need more information?
A burning question, a specific request, a great project to share with us? Get in touch, our teams are here to help!










